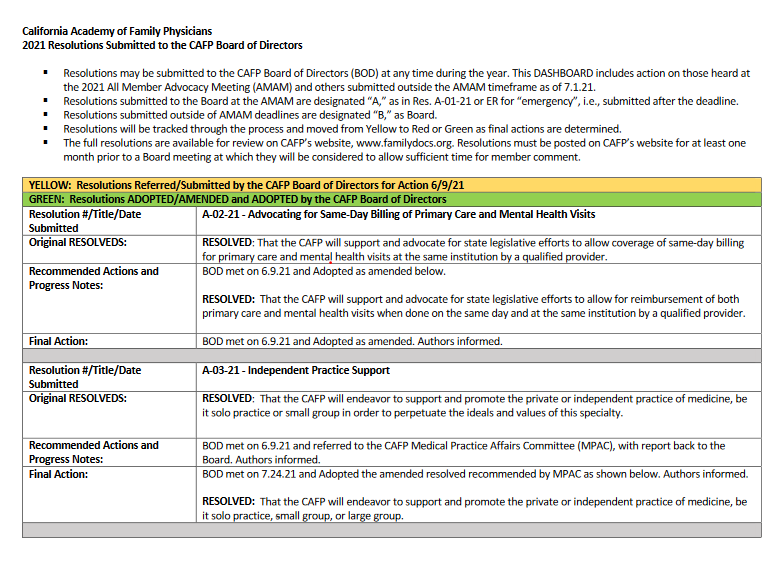
CAFP has updated the chart reflecting the actions taken by the Board on all resolutions which has been posted here. As a reminder, the chart of actions taken is updated after each quarterly meeting of the Board and posted to the website and shared with members via the Academy in Action newsletter. While the majority of […]